
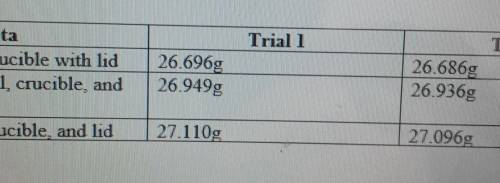
The actual yield of Magnesium Oxide in trial one is 0.414g, and in trial two it's 0.410.
With this information, what is the theoretical yield of MgO (in both trials) with Magnesium as the limiting reactant.
What is the percent yield of MgO for each trial.
What is the average percent yield of MgO for both trials.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
The actual yield of Magnesium Oxide in trial one is 0.414g, and in trial two it's 0.410.
With this...
Questions



English, 07.10.2019 05:30

History, 07.10.2019 05:30


Geography, 07.10.2019 05:30

Chemistry, 07.10.2019 05:30

Mathematics, 07.10.2019 05:30


Mathematics, 07.10.2019 05:30






History, 07.10.2019 05:30



English, 07.10.2019 05:30




