
Chemistry, 28.12.2020 08:00 Aliciaonfleek
A gaseous mixture of 1.0 mol of 12 and 1.0 mol of H2 was placed in an empty vessel that has a
volume of 2.0 L. The system was allowed to reach dynamic equilibrium at 300°C.
a) State briefly what happened to the initial concentration of 12 and H2 as the reaction reaches
equilibrium
b) Does the reaction stoop at equilibrium? Why?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:10
Amonoprotic acid is an acid that donates a single proton to the solution. suppose you have 0.140 g of a monoprotic acid dissolved in 35.0 ml of water. this solution is then neutralized with 14.5 ml of 0.110 m naoh. what is the molar mass of the acid?
Answers: 1

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

You know the right answer?
A gaseous mixture of 1.0 mol of 12 and 1.0 mol of H2 was placed in an empty vessel that has a
volum...
Questions

Mathematics, 06.06.2020 10:58

Mathematics, 06.06.2020 10:58


English, 06.06.2020 10:58


Mathematics, 06.06.2020 10:58

Computers and Technology, 06.06.2020 10:58

Mathematics, 06.06.2020 10:58



Mathematics, 06.06.2020 10:58

Social Studies, 06.06.2020 10:58


English, 06.06.2020 10:58



Mathematics, 06.06.2020 10:58

Mathematics, 06.06.2020 10:58

Mathematics, 06.06.2020 10:58

Mathematics, 06.06.2020 10:58

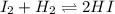
![\frac{[HI]^2}{[I_2][H_2]}](/tpl/images/1008/4126/2a174.png)


