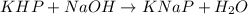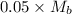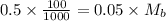Chemistry, 28.12.2020 15:50 zfghiiooi5
Someone help me please im stuck on a equation
3. Rearrange the titration calculation to find the Mb in a solution. (* Mb = molarity of the basic solution )
(~0.3 mL) of indicator to the KHP
solution.
(~ 0.5 mL or less) of NaOH
The moles of acid= 0.5 x 100/1000=0.05 Mb = (0.5/55)x1000=0.9

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
Someone help me please im stuck on a equation
3. Rearrange the titration calculation to find the Mb...
Questions

Mathematics, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01


Mathematics, 20.10.2020 03:01


Computers and Technology, 20.10.2020 03:01









English, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01




Social Studies, 20.10.2020 03:01