Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1


Chemistry, 23.06.2019 11:30
The density of e85 fuel is 0.801 g/ml. what is the mass of 1.00 gallon of the fuel? (1 gal. = 3.785 l)
Answers: 3
You know the right answer?
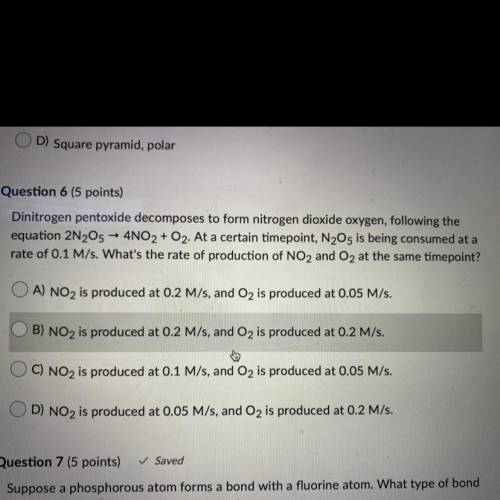
dinitrogen pentoxide decomposes to form nitrogen dioxide oxygen, following the equation 2N2O5 ->...
Questions

Mathematics, 28.03.2021 14:00

English, 28.03.2021 14:00


Medicine, 28.03.2021 14:00



Mathematics, 28.03.2021 14:00

English, 28.03.2021 14:00


Mathematics, 28.03.2021 14:00


Mathematics, 28.03.2021 14:00


Physics, 28.03.2021 14:00

English, 28.03.2021 14:00



Mathematics, 28.03.2021 14:00


English, 28.03.2021 14:00