
Chemistry, 29.12.2020 14:00 CameronVand21
What would happen to the rate of a reaction with rate law rate = k [NO]^2[H2] if
the concentration of NO were halved?
A. The rate would be four times larger.
B. The rate would also be halved.
C. The rate would be one-fourth.
D. The rate would be doubled.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 23.06.2019 06:30
(04.01 lc) which of the following is true about science? (5 points) select one: a. it is not influenced by social conditions. b. it is not determined by external local factors. c. political conditions are unable to influence it. d. economic concerns may prevent it from solving problems.
Answers: 1
You know the right answer?
What would happen to the rate of a reaction with rate law rate = k [NO]^2[H2] if
the concentration...
Questions

Chemistry, 29.03.2021 23:00

Mathematics, 29.03.2021 23:00


English, 29.03.2021 23:00

History, 29.03.2021 23:00

Business, 29.03.2021 23:00


History, 29.03.2021 23:00



English, 29.03.2021 23:00



Mathematics, 29.03.2021 23:00


Mathematics, 29.03.2021 23:00

Health, 29.03.2021 23:00

Mathematics, 29.03.2021 23:00

Mathematics, 29.03.2021 23:00

History, 29.03.2021 23:00

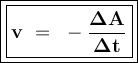
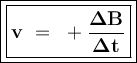
![\tt r_2=k[\dfrac{1}{2}No]^2[H_2]\\\\r_2=\dfrac{1}{4}k.[No]^2[H_2]\\\\r_2=\dfrac{1}{4}r_1](/tpl/images/1009/0129/44ffe.png)


