
Chemistry, 31.12.2020 01:00 maddie53116
Use your knowledge of modern atomic theory and bonding to explain each of the following experimental observations. (Your explanations should be specific.) Refer to both substances when comparing. Within a family such as the alkaline earth metals, the ionic radius increases as the atomic number increases. The radius of the bromine atom is smaller than the radius of the bromide ion, Br-1. The first ionization energy of aluminum is lower than the first ionization energy of magnesium.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1
You know the right answer?
Use your knowledge of modern atomic theory and bonding to explain each of the following experimental...
Questions

Mathematics, 28.04.2021 04:10

Biology, 28.04.2021 04:10

Mathematics, 28.04.2021 04:10

Mathematics, 28.04.2021 04:10


Mathematics, 28.04.2021 04:10

History, 28.04.2021 04:10


English, 28.04.2021 04:10



Social Studies, 28.04.2021 04:10

Health, 28.04.2021 04:10

History, 28.04.2021 04:10




Mathematics, 28.04.2021 04:10


Mathematics, 28.04.2021 04:10

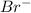 ion, the configuration of bromine earlier was
ion, the configuration of bromine earlier was 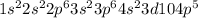 but on addition of the electron the p shell will now have 6 electrons resulting in
but on addition of the electron the p shell will now have 6 electrons resulting in 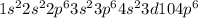 the newly added electron repels the former neutral orientation and as a result of repulsion the size increases.
the newly added electron repels the former neutral orientation and as a result of repulsion the size increases. 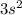 orbital whereas Aluminum has one electron in the 3p orbitals, fully filled 3s orbitals has strongly held by the nucleus in case of Mg whereas 3p orbitals is shielded from the nucleus therefore it becomes easy to take out 3p electron that 3s electron.
orbital whereas Aluminum has one electron in the 3p orbitals, fully filled 3s orbitals has strongly held by the nucleus in case of Mg whereas 3p orbitals is shielded from the nucleus therefore it becomes easy to take out 3p electron that 3s electron. 

