temperature will change from

Chemistry, 31.12.2020 02:20 katelynalivia
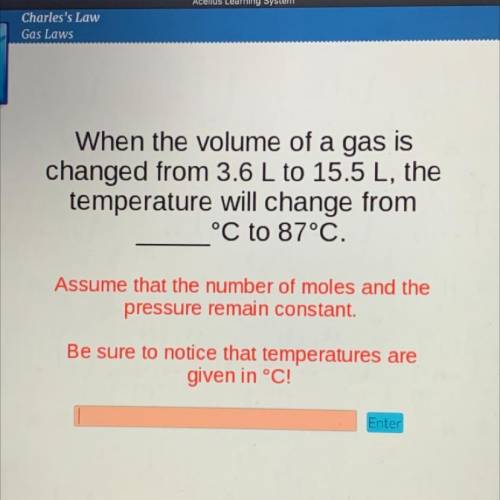
When the volume of a gas is
changed from 3.6 L to 15.5 L, the
temperature will change from
°C to 87°C.
Assume that the number of moles and the
pressure remain constant.
Be sure to notice that temperatures are
given in °C!

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3
You know the right answer?
When the volume of a gas is
changed from 3.6 L to 15.5 L, the
temperature will change from
temperature will change from
Questions

Mathematics, 08.06.2020 23:57

Mathematics, 08.06.2020 23:57





Mathematics, 08.06.2020 23:57

English, 08.06.2020 23:57



Mathematics, 08.06.2020 23:57

Mathematics, 08.06.2020 23:57

Mathematics, 08.06.2020 23:57


Mathematics, 08.06.2020 23:57


Mathematics, 08.06.2020 23:57


Geography, 08.06.2020 23:57



