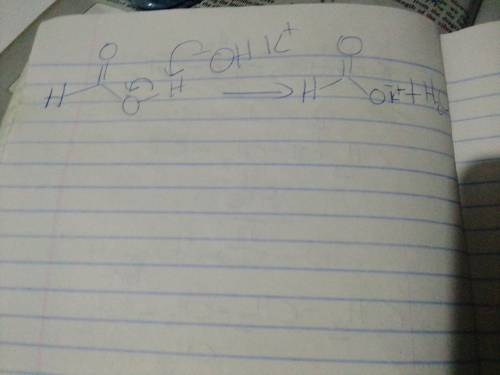Chemistry, 31.12.2020 19:00 sassycutie523
When formic acid is treated with potassium hydroxide (KOH), an acid-base reaction occurs, forming a carboxylate ion. For the mechanism, draw the curved arrows as needed. Include lone pairs and charges in your answer. Do not draw out any hydrogen explicitly in your products.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
When formic acid is treated with potassium hydroxide (KOH), an acid-base reaction occurs, forming a...
Questions


Mathematics, 02.11.2020 23:30

Biology, 02.11.2020 23:30



Physics, 02.11.2020 23:30

Geography, 02.11.2020 23:30


Mathematics, 02.11.2020 23:30



Mathematics, 02.11.2020 23:30


Mathematics, 02.11.2020 23:30

Mathematics, 02.11.2020 23:30




Social Studies, 02.11.2020 23:30

Chemistry, 02.11.2020 23:30