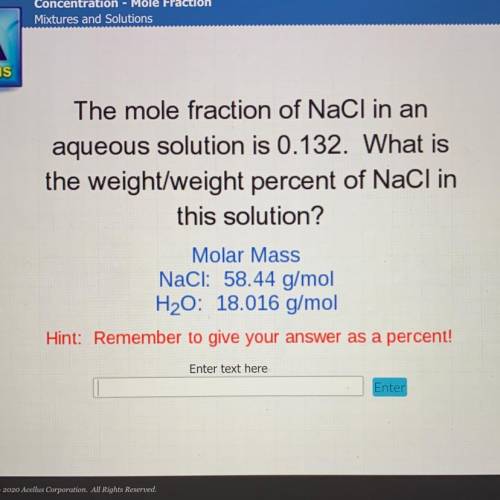
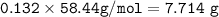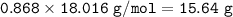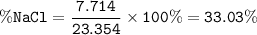The mole fraction of NaCl in an
aqueous solution is 0.132. What is
the weight/weight percent...


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

You know the right answer?
Questions

Biology, 07.04.2020 22:33


History, 07.04.2020 22:33






History, 07.04.2020 22:33



Biology, 07.04.2020 22:33


English, 07.04.2020 22:33



Chemistry, 07.04.2020 22:33