
Chemistry, 02.01.2021 02:10 cdradlet2001
SO2Cl2 rightarrow SO2 (g) + Cl2 (g)At 600 K, SO2Cl2 will decompose to form sulfure dioxide and chlorine gas via the above equation. If the reaction is found to be first order overall, which of the following will cause an increase in he half life of SO2CL2?A. Increasing the initial concentration of SO2,CI2.B. Increasing the temperature at which the reaction occurs. C. Decreasing the overall pressure in the container. D. None of these will increase the half life.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle i need : ( asap i go it never mind
Answers: 2

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2
You know the right answer?
SO2Cl2 rightarrow SO2 (g) + Cl2 (g)At 600 K, SO2Cl2 will decompose to form sulfure dioxide and chlor...
Questions

Physics, 25.08.2019 01:30

Biology, 25.08.2019 01:30


Health, 25.08.2019 01:30

Social Studies, 25.08.2019 01:30

Mathematics, 25.08.2019 01:30

Mathematics, 25.08.2019 01:30


History, 25.08.2019 01:30



Mathematics, 25.08.2019 01:30

English, 25.08.2019 01:30

History, 25.08.2019 01:30

History, 25.08.2019 01:30


Mathematics, 25.08.2019 01:30

Physics, 25.08.2019 01:30


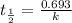
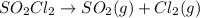 following first order kinetics, none of the factors will increase the half life.
following first order kinetics, none of the factors will increase the half life.

