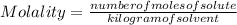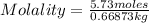Chemistry, 02.01.2021 16:30 jessica2138
Calculate the molality of a 5.73 M ethanol (C2H5OH) solution whose density is 0.9327 g/mL.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1


Chemistry, 23.06.2019 12:30
17) large amounts of very important metal titanium are made by reacting magnesium metal with titanium tetrachloride. titanium metal and magnesium chloride are produced. a) write the balanced equation for this reaction. b) how many kilograms of magnesium are required to produce 1.00 kilograms of titanium? ( show work, .)
Answers: 1
You know the right answer?
Calculate the molality of a 5.73 M ethanol (C2H5OH) solution whose density is 0.9327 g/mL....
Questions

English, 05.12.2020 17:50


Computers and Technology, 05.12.2020 17:50


Mathematics, 05.12.2020 17:50

Mathematics, 05.12.2020 17:50

Business, 05.12.2020 17:50

Mathematics, 05.12.2020 17:50


English, 05.12.2020 17:50


Mathematics, 05.12.2020 17:50


Computers and Technology, 05.12.2020 17:50

Mathematics, 05.12.2020 17:50

Biology, 05.12.2020 17:50


Mathematics, 05.12.2020 17:50

Mathematics, 05.12.2020 17:50

 .
.