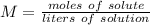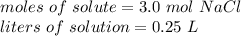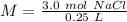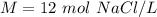Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
What is the molarity of a solution made from dissolving 3.0 moles of NaCl in 250 mL of solution?
3....
Questions

Biology, 12.10.2019 03:30

Biology, 12.10.2019 03:30


Mathematics, 12.10.2019 03:30

Mathematics, 12.10.2019 03:30



Mathematics, 12.10.2019 03:50




Mathematics, 12.10.2019 03:50

Mathematics, 12.10.2019 03:50


Chemistry, 12.10.2019 03:50

Business, 12.10.2019 03:50


Chemistry, 12.10.2019 03:50

Mathematics, 12.10.2019 03:50