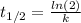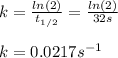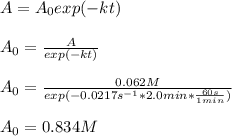Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
The half-life for a first-order reaction is 32 s. What was the original concentration if, after 2.0...
Questions

History, 30.11.2020 18:50

Health, 30.11.2020 18:50

Mathematics, 30.11.2020 18:50


History, 30.11.2020 18:50






Mathematics, 30.11.2020 18:50

English, 30.11.2020 18:50

History, 30.11.2020 18:50

English, 30.11.2020 18:50

Computers and Technology, 30.11.2020 18:50


History, 30.11.2020 18:50

History, 30.11.2020 18:50

History, 30.11.2020 18:50