

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
4. (05.06 LC)
The actual yield of a product in a reaction was measured as 2.80 g. If the theoretica...
Questions




Mathematics, 19.04.2021 20:20

Computers and Technology, 19.04.2021 20:20

Biology, 19.04.2021 20:20



History, 19.04.2021 20:20

Law, 19.04.2021 20:20


Mathematics, 19.04.2021 20:20

Mathematics, 19.04.2021 20:20




Social Studies, 19.04.2021 20:20

Spanish, 19.04.2021 20:20

Mathematics, 19.04.2021 20:20

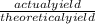 ×100). So, simply put 2.80 over 3.12 and whatever you get multiply times 100 to make it a percentage.
×100). So, simply put 2.80 over 3.12 and whatever you get multiply times 100 to make it a percentage. 

