
Chemistry, 04.01.2021 20:30 deidaraXneji
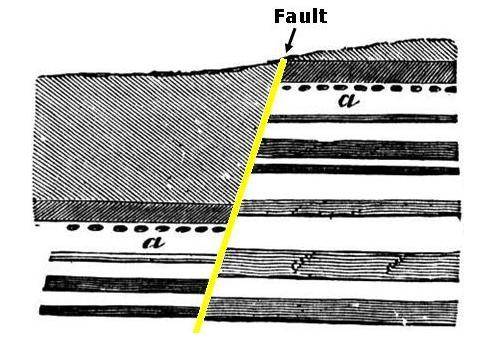
The image below shows a stack of rock layers exposed in a cliffside. These layers have a fault running through them. A fault is a crack along which rocks move.
When these layers first formed long ago, there was no fault present. At some time later, tectonic forces cracked the layers, forming the fault. Then, the rocks on either side of the fault moved. Using the layer marked a as a guide, determine how the rocks moved along the fault.
A.
The rocks on both sides of the fault moved down the same distance.
B.
The rocks on the left side moved up, and the rocks on the right side moved down.
C.
The rocks on the right side moved up, and the rocks on the left side moved down.
D.
The rocks on both sides of the fault moved up the same distance.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
You know the right answer?
The image below shows a stack of rock layers exposed in a cliffside. These layers have a fault runni...
Questions





Mathematics, 15.07.2020 01:01

Mathematics, 15.07.2020 01:01







English, 15.07.2020 01:01

Mathematics, 15.07.2020 01:01



Mathematics, 15.07.2020 01:01






