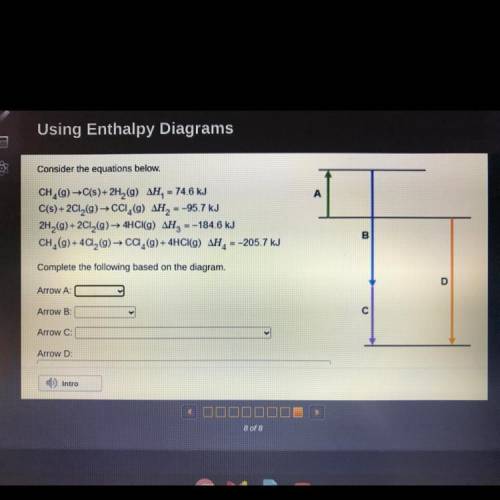
Consider the equations below.
CH, (9) →C(s)+ 2H, (9) AH, – 74.6 kJ
C(s) + 2CI,(9)→ cCi, () AH...

Chemistry, 05.01.2021 01:50 breannamiller0822
Consider the equations below.
CH, (9) →C(s)+ 2H, (9) AH, – 74.6 kJ
C(s) + 2CI,(9)→ cCi, () AH, =-95.7 kJ
2H,(g) + 2CI,(9)→ 4HCI(G) AH, =-184.6 kJ
CH, (9) + 4Ca, (9) → Ca,(9) + 4HCI(9) AH, =-205.7 kJ
Complete the following based on the diagram.
Arrow A:
Arrow B:
Arrow C:
Arrow D:

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2


You know the right answer?
Questions

English, 05.04.2021 14:20


Mathematics, 05.04.2021 14:20

Chemistry, 05.04.2021 14:20

English, 05.04.2021 14:20

English, 05.04.2021 14:20

English, 05.04.2021 14:30

Health, 05.04.2021 14:30


Mathematics, 05.04.2021 14:30

English, 05.04.2021 14:30

English, 05.04.2021 14:30

English, 05.04.2021 14:30

Business, 05.04.2021 14:30


Mathematics, 05.04.2021 14:30


Mathematics, 05.04.2021 14:30

History, 05.04.2021 14:30



