
Consider the following equilibrium: 2SO^2(g) + O2(9) = 2 SO3^(g)
1. What is equal at equilibrium?
2. What would happen to the forward rate if some 0were removed from this equilibrium?
3. Explain why, in terms of collision theory.
4. Would the reaction still be at equilibrium at this point?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
Consider the following equilibrium: 2SO^2(g) + O2(9) = 2 SO3^(g)
1. What is equal at equilibrium?
Questions


Advanced Placement (AP), 01.08.2020 23:01





Mathematics, 01.08.2020 23:01






Advanced Placement (AP), 01.08.2020 23:01




Mathematics, 01.08.2020 23:01



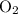 is removed from the mixture. The reason is that collisions between
is removed from the mixture. The reason is that collisions between 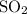 molecules and
molecules and 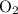 molecules would become less frequent.
molecules would become less frequent. 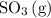 to the system, the backward reaction would have broken down the same amount of
to the system, the backward reaction would have broken down the same amount of 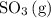 . So is the case for
. So is the case for 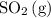 and
and 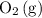 .
.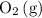 molecules would be present in the mixture, there would be fewer collisions (fruitful or not) between
molecules would be present in the mixture, there would be fewer collisions (fruitful or not) between 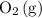 molecules in unit time. Even if the percentage of fruitful collisions stays the same, there would fewer fruitful collisions in unit time. It would thus appear that the forward reaction has become slower.
molecules in unit time. Even if the percentage of fruitful collisions stays the same, there would fewer fruitful collisions in unit time. It would thus appear that the forward reaction has become slower. 

