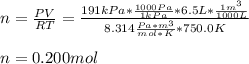Chemistry, 05.01.2021 16:20 parkerfreeze
If an instant pot has an internal pressure of 191.0 kPa, temperature of 750.0 K and a volume of 6.5 L, how many moles of gas would be contained inside the instant pot?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3
You know the right answer?
If an instant pot has an internal pressure of 191.0 kPa, temperature of 750.0 K and a volume of 6.5...
Questions


Computers and Technology, 30.05.2020 19:01


English, 30.05.2020 19:01

History, 30.05.2020 19:01






Mathematics, 30.05.2020 19:01

Mathematics, 30.05.2020 19:01







Mathematics, 30.05.2020 19:01