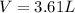Chemistry, 05.01.2021 16:50 thegent3473
Calculate the volume of oxygen at NTP obtained by decomposing 12.26g of KCLO3(at wt. K=39.1, Cl=35.5 and O = 16)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
Calculate the volume of oxygen at NTP obtained by decomposing 12.26g of KCLO3(at wt. K=39.1, Cl=35.5...
Questions




Mathematics, 02.08.2019 12:00

History, 02.08.2019 12:00

Biology, 02.08.2019 12:00


History, 02.08.2019 12:00

History, 02.08.2019 12:00

History, 02.08.2019 12:00

History, 02.08.2019 12:00


History, 02.08.2019 12:00


Geography, 02.08.2019 12:00

Computers and Technology, 02.08.2019 12:00

Geography, 02.08.2019 12:00

Physics, 02.08.2019 12:00

Chemistry, 02.08.2019 12:00

English, 02.08.2019 12:00

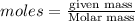
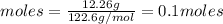
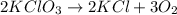
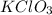 produce = 3 moles of
produce = 3 moles of 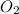
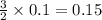 moles of
moles of