
Chemistry, 05.01.2021 16:40 Skylar4483
According to this balanced chemical equation,
what volume of C2H2 is required to form 40.0L
of CO2?
2C2H2(g) + 502(g) → 2H2O(g) + 4CO2(g)
A. 20.0L
B. 44.8L
C. 80.0L
D. 100 L

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:20
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases.as altitude increases, air density increases.air pressure and density are lowest at sea level.denser air exerts more pressure than less dense air.
Answers: 2

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2
You know the right answer?
According to this balanced chemical equation,
what volume of C2H2 is required to form 40.0L
Questions

History, 19.08.2019 03:30


Social Studies, 19.08.2019 03:30



Biology, 19.08.2019 03:30


Biology, 19.08.2019 03:30

Biology, 19.08.2019 03:30

Geography, 19.08.2019 03:30

Social Studies, 19.08.2019 03:30

History, 19.08.2019 03:30

Mathematics, 19.08.2019 03:30







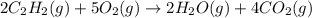
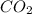 is formed by = 2 L of
is formed by = 2 L of 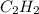
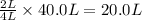 of
of 

