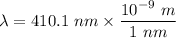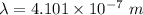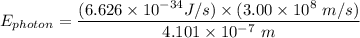The wavelength of the violet light emitted from a hydrogen atom is 410.1 nm. This light is a result of electronic transitions between the n = 5 and n = 2 energy levels. How much higher in energy is the n = 5 energy level than the n = 2 energy level?
Select one:
a. 3.000 x 108 J
b. 1.114 x 10-14 J
c. 2.436 x 10-18 J
d. 1.616 x 10-36 J
e. 4.847 x 10-19 J

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:10
Which of these will change if the air in aclosed bottle is heated? abcdthe mass of the airthe composition of the airthe air pressure in the bottlethe number of air molecules in the bottle
Answers: 3

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2
You know the right answer?
The wavelength of the violet light emitted from a hydrogen atom is 410.1 nm. This light is a result...
Questions

Mathematics, 10.04.2020 23:36


English, 10.04.2020 23:38

Mathematics, 10.04.2020 23:39




History, 10.04.2020 23:45


Chemistry, 10.04.2020 23:45










Computers and Technology, 10.04.2020 23:46

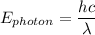
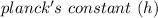 = 6.626 * 10 ^{-34} J.s
= 6.626 * 10 ^{-34} J.s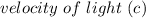 = 3.00 * 10^8 m/s
= 3.00 * 10^8 m/s