
Chemistry, 05.01.2021 20:30 mubasharshafiq1583
Gas cloud 1 is likely to form a star. Gas cloud 2 is not. Based on this information, match the given conditions with each cloud. Its temperature stays well below 14 million Kelvin. Its volume shrinks and density increases due to gravity. Its temperature surpasses 14 million Kelvin. It is spread out, with a greater volume and lesser concentration of elements. Its hydrogen atoms shed their electrons. Its hydrogen atoms retain their electrons.
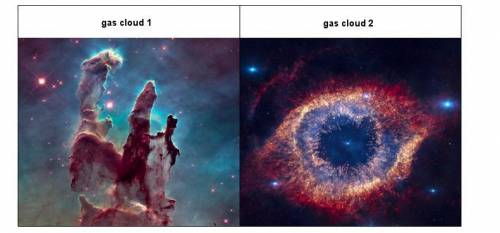

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1


Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2
You know the right answer?
Gas cloud 1 is likely to form a star. Gas cloud 2 is not. Based on this information, match the given...
Questions









Mathematics, 28.11.2019 22:31



Mathematics, 28.11.2019 22:31






History, 28.11.2019 22:31

History, 28.11.2019 22:31



