
Chemistry, 06.01.2021 01:40 eshavaggar
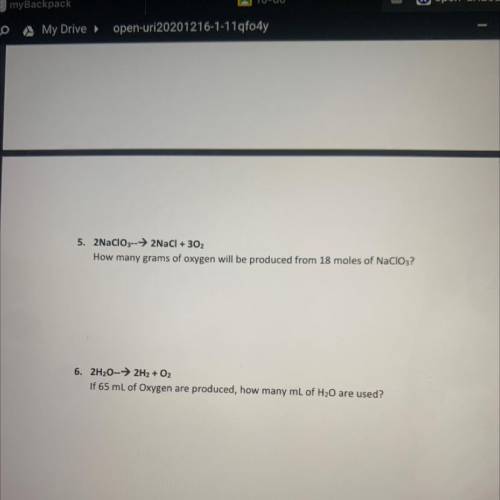
5. 2NaClO3-->2NaCl + 302
How many grams of oxygen will be produced from 18 moles of NaClO3?
6. 2H20-→ 2H2 + O2
If 65 mL of Oxygen are produced, how many mL of H20 are used?
Show all work

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

You know the right answer?
5. 2NaClO3-->2NaCl + 302
How many grams of oxygen will be produced from 18 moles of NaClO3?
Questions

Mathematics, 03.08.2020 17:01

Mathematics, 03.08.2020 18:01


Mathematics, 03.08.2020 18:01



Mathematics, 03.08.2020 18:01

History, 03.08.2020 18:01

Chemistry, 03.08.2020 18:01

Biology, 03.08.2020 18:01

Engineering, 03.08.2020 18:01


Mathematics, 03.08.2020 18:01

Mathematics, 03.08.2020 18:01


Business, 03.08.2020 18:01



History, 03.08.2020 18:01



