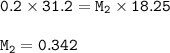Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
Please help me A 0.200 M NaOH solution was used to titrate a 18.25 mL HE
solution. The endpoint was...
Questions



Mathematics, 26.03.2020 19:48


Chemistry, 26.03.2020 19:48



English, 26.03.2020 19:49


Mathematics, 26.03.2020 19:49



Mathematics, 26.03.2020 19:49


Mathematics, 26.03.2020 19:49



Mathematics, 26.03.2020 19:50

History, 26.03.2020 19:50