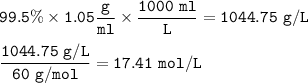Chemistry, 06.01.2021 04:00 Falconpride4079
If a concentrated solution of acetic acid is 99.5 % HC 2 H 3 O 2 and has a density of 1.05 g/mL, what is the concentration of this acid in moles per litre?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1


Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
If a concentrated solution of acetic acid is 99.5 % HC 2 H 3 O 2 and has a density of 1.05 g/mL, wha...
Questions

Mathematics, 17.12.2020 18:00


Chemistry, 17.12.2020 18:00

Mathematics, 17.12.2020 18:00



English, 17.12.2020 18:00



Computers and Technology, 17.12.2020 18:00



Chemistry, 17.12.2020 18:00



Business, 17.12.2020 18:00

Chemistry, 17.12.2020 18:00

Mathematics, 17.12.2020 18:00

Computers and Technology, 17.12.2020 18:00

Mathematics, 17.12.2020 18:00