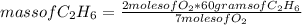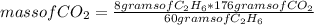Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2


Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
In a reaction, 2mol of oxygen reacts with 8g of ethane to produce carbon dioxide and water, calculat...
Questions


English, 14.03.2020 06:19



Social Studies, 14.03.2020 06:20


Biology, 14.03.2020 06:20

Mathematics, 14.03.2020 06:20




Mathematics, 14.03.2020 06:20


Mathematics, 14.03.2020 06:21

Health, 14.03.2020 06:21





Arts, 14.03.2020 06:23