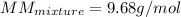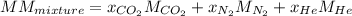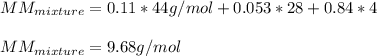Chemistry, 07.01.2021 17:00 quintasiahaskin
The gas in the discharge cell of a laser contains (in mole percent) 11% CO2, 5.3% N2, and 84% He. (a) What is the molar mass of this mixture

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1
You know the right answer?
The gas in the discharge cell of a laser contains (in mole percent) 11% CO2, 5.3% N2, and 84% He. (a...
Questions





Mathematics, 02.11.2020 02:00



Advanced Placement (AP), 02.11.2020 02:00





Mathematics, 02.11.2020 02:00


Mathematics, 02.11.2020 02:00

Mathematics, 02.11.2020 02:00

Advanced Placement (AP), 02.11.2020 02:00



Spanish, 02.11.2020 02:00