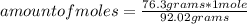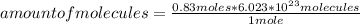Chemistry, 07.01.2021 18:30 orangeicecream
How many molecules of N2O4 are in 76.3g N2O4? The molar mass of N2O4 is 92.02 g/mol.
a. 4.59 × 10^25 N2O4 molecules
b. 5.54 × 10^25 N2O4 molecules
c. 7.26 × 10^23 N2O4 molecules
d. 1.38 × 10^24 N2O4 molecules
e. 4.99 × 10^23 N2O4 molecules

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1


Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1
You know the right answer?
How many molecules of N2O4 are in 76.3g N2O4? The molar mass of N2O4 is 92.02 g/mol.
a. 4.59 × 10^2...
Questions


Mathematics, 03.03.2021 18:30

Mathematics, 03.03.2021 18:30

Mathematics, 03.03.2021 18:30



World Languages, 03.03.2021 18:30

Chemistry, 03.03.2021 18:30

Mathematics, 03.03.2021 18:30

Chemistry, 03.03.2021 18:30


Mathematics, 03.03.2021 18:30

Chemistry, 03.03.2021 18:30



Mathematics, 03.03.2021 18:30

Mathematics, 03.03.2021 18:30