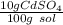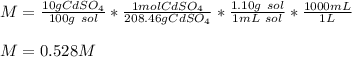Chemistry, 07.01.2021 18:30 arieltaylor3924
What is the molarity of a solution of 10% by mass cadmium sulfate, CdSO4 (molar mass = 208.46 g/mol) by mass? The density of the solution is 1.10 g/mL.
a. 0.528 M
b. 0.436 M
c. 0.479 M
d. 0.048 M
e. 22.9 M

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1


Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1
You know the right answer?
What is the molarity of a solution of 10% by mass cadmium sulfate, CdSO4 (molar mass = 208.46 g/mol)...
Questions

History, 20.01.2021 04:30

Computers and Technology, 20.01.2021 04:30

History, 20.01.2021 04:30





Chemistry, 20.01.2021 04:30



Mathematics, 20.01.2021 04:30

Mathematics, 20.01.2021 04:30






Mathematics, 20.01.2021 04:30

Mathematics, 20.01.2021 04:30

English, 20.01.2021 04:30