
Chemistry, 08.01.2021 01:00 EllaLovesAnime
Use the following reaction:
CuCl2 + 2 NaNO3 → Cu(NO3)2 + 2 NaCl
If 90.0 grams of copper (II) chloride react with 120.0 grams of sodium nitrate, how much sodium chloride can be formed (in grams)?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

You know the right answer?
Use the following reaction:
CuCl2 + 2 NaNO3 → Cu(NO3)2 + 2 NaCl
If 90.0 grams of copper...
If 90.0 grams of copper...
Questions

Mathematics, 09.10.2019 08:50



Mathematics, 09.10.2019 08:50

Chemistry, 09.10.2019 08:50


History, 09.10.2019 08:50

Computers and Technology, 09.10.2019 08:50

English, 09.10.2019 08:50

Mathematics, 09.10.2019 08:50

History, 09.10.2019 08:50


History, 09.10.2019 08:50





Mathematics, 09.10.2019 08:50

Chemistry, 09.10.2019 08:50

Mathematics, 09.10.2019 08:50

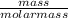
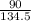 = 0.67mole
= 0.67mole 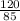 = 1.41mole
= 1.41mole 

