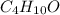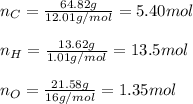Chemistry, 08.01.2021 01:50 cheycheybabygirl01
A compound is found to contain 64.80 % carbon, 13.62 % hydrogen, and 21.58 % oxygen by weight. To answer the questions, enter the elements in the order presented above. 1. What is the empirical formula for this compound

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2


Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
A compound is found to contain 64.80 % carbon, 13.62 % hydrogen, and 21.58 % oxygen by weight. To an...
Questions


Chemistry, 02.03.2021 04:50





Health, 02.03.2021 04:50

Mathematics, 02.03.2021 05:00


Biology, 02.03.2021 05:00

English, 02.03.2021 05:00

English, 02.03.2021 05:00

Mathematics, 02.03.2021 05:00


Mathematics, 02.03.2021 05:00

Mathematics, 02.03.2021 05:00

English, 02.03.2021 05:00


Mathematics, 02.03.2021 05:00