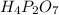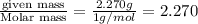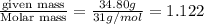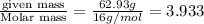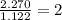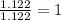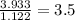Chemistry, 08.01.2021 02:20 goldenrizo
A compound is found to contain 2.270 % hydrogen, 34.80 % phosphorus, and 62.93 % oxygen by mass. What is the empirical formula for this compound

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
A compound is found to contain 2.270 % hydrogen, 34.80 % phosphorus, and 62.93 % oxygen by mass. Wha...
Questions


Mathematics, 30.08.2020 07:01

Mathematics, 30.08.2020 07:01


Mathematics, 30.08.2020 07:01

Mathematics, 30.08.2020 07:01

Physics, 30.08.2020 07:01

Physics, 30.08.2020 07:01


Mathematics, 30.08.2020 07:01


Advanced Placement (AP), 30.08.2020 07:01

Business, 30.08.2020 07:01


Social Studies, 30.08.2020 07:01

Mathematics, 30.08.2020 07:01


Chemistry, 30.08.2020 07:01

Geography, 30.08.2020 07:01