
Chemistry, 08.01.2021 02:40 elondamason
Suppose a lab procedure calls for 0.75 L of a 0.25 M CaCl2 solution. How much of a 10.0 M stock solution do we dilute? Where the diluted solution is #2 (the numerator)
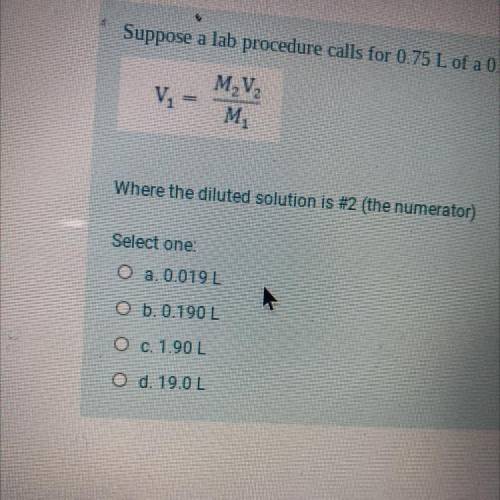

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
Suppose a lab procedure calls for 0.75 L of a 0.25 M CaCl2 solution. How much of a 10.0 M stock solu...
Questions


Mathematics, 04.07.2019 01:30


Mathematics, 04.07.2019 01:30

Mathematics, 04.07.2019 01:30



Computers and Technology, 04.07.2019 01:30



Computers and Technology, 04.07.2019 01:30


Mathematics, 04.07.2019 01:30









