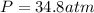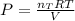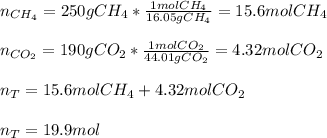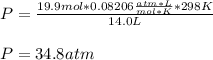Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
a 14.0 l tank contains 250 g of methane (CH4) gas at 27 atm at 298 K. Accidentally, 190 g of CO2 was...
Questions




English, 18.12.2019 17:31

History, 18.12.2019 17:31


History, 18.12.2019 17:31





Mathematics, 18.12.2019 17:31




History, 18.12.2019 17:31

Physics, 18.12.2019 17:31