
Chemistry, 20.11.2019 19:31 Masielovebug
Heat is added to a 200.-gram sample of h2o(s) to melt the sample at 0°c. then the resulting h2o ( ) is heated to a final temperature of 65°c. determine the total amount of heat required to completely melt the sample. heat is added to a 200.-gram sample of h2o(s) to melt the sample at 0°c. then the resulting h2o ( ) is heated to a final temperature of 65°c. determine the total amount of heat required to completely melt the sample.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1

Chemistry, 23.06.2019 19:00
In which of the following is concentration expressed in percent by volume
Answers: 2
You know the right answer?
Heat is added to a 200.-gram sample of h2o(s) to melt the sample at 0°c. then the resulting h2o ( )...
Questions




Mathematics, 09.12.2020 23:30

Mathematics, 09.12.2020 23:30

Geography, 09.12.2020 23:30


Mathematics, 09.12.2020 23:30

History, 09.12.2020 23:30

History, 09.12.2020 23:30


English, 09.12.2020 23:30

SAT, 09.12.2020 23:30

English, 09.12.2020 23:30

Mathematics, 09.12.2020 23:30


Mathematics, 09.12.2020 23:30

Mathematics, 09.12.2020 23:30


History, 09.12.2020 23:30

 of energy is required to melt the sample completely.
of energy is required to melt the sample completely.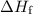 .
. is converted to water at
is converted to water at 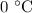 .
. ...... (1)
...... (1) 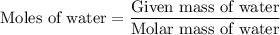 ...... (2)
...... (2) 
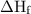 in equation (1).
in equation (1).
 .
. …… (3)
…… (3)  is the temperature change.
is the temperature change.
 for
for  for c in equation (3).
for c in equation (3).


 ...... (4)
...... (4)  ,and
,and 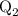 are the values of energies calculated in first and second step respectively.
are the values of energies calculated in first and second step respectively.


