
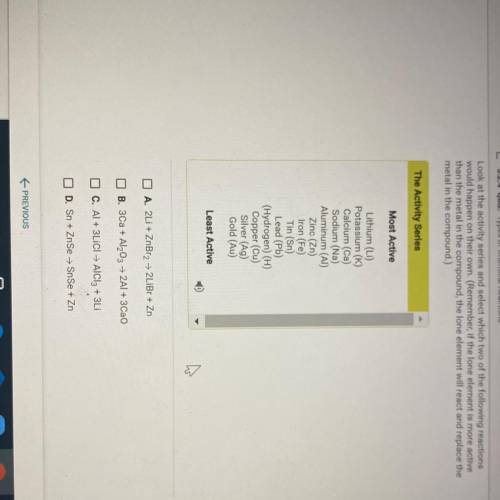
Look at the activity series and select which two of the following reactions
would happen on their own. (Remember, if the lone element is more active
than the metal in the compound, the lone element will react and replace the
metal in the compound.)
The Activity Series
Most Active
Lithium (Li)
Potassium (K)
Calcium (Ca)
Sodium (Na)
Aluminum (AI)
Zinc (Zn)
Iron (Fe)
Tin (Sn)
Lead (Pb)
(Hydrogen) (H)
Copper (Cu)
Silver (Ag)
Gold (Au)
Least Active
O A. 2Li + ZnBr2 + 2LiBr + Zn
B. 3Ca+ Al2O3 + 2Al + 3Cao
C. Al +3LICI - AlCl3 + 3Li
D. Sn + ZnSe → SnSe + Zn

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1
You know the right answer?
Look at the activity series and select which two of the following reactions
would happen on their o...
Questions

Mathematics, 09.09.2021 01:40


Biology, 09.09.2021 01:40





Chemistry, 09.09.2021 01:40


Mathematics, 09.09.2021 01:40

Arts, 09.09.2021 01:40




Chemistry, 09.09.2021 01:50


Mathematics, 09.09.2021 01:50





