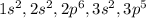Chemistry, 08.01.2021 17:50 izaiahfieods
A neutral atom has the following electronic configuration: 1s2 2s2 2p6 3s2 3p5 How many protons are in the atomic nucleus and to which group of the periodic table does this element belong?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
You know the right answer?
A neutral atom has the following electronic configuration: 1s2 2s2 2p6 3s2 3p5
How many protons are...
Questions

Mathematics, 02.02.2021 22:00

Mathematics, 02.02.2021 22:00

Arts, 02.02.2021 22:00


English, 02.02.2021 22:00

Mathematics, 02.02.2021 22:00


English, 02.02.2021 22:00

Biology, 02.02.2021 22:00

Mathematics, 02.02.2021 22:00

Mathematics, 02.02.2021 22:00

Mathematics, 02.02.2021 22:00

English, 02.02.2021 22:00

Mathematics, 02.02.2021 22:00

Engineering, 02.02.2021 22:00

English, 02.02.2021 22:00


Mathematics, 02.02.2021 22:00


History, 02.02.2021 22:00