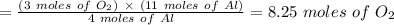Chemistry, 08.01.2021 18:40 maytce7237
Using the balanced chemical equation below. 2Al2O3 --> 4Al + 3O2 How many moles of oxygen are produced if 11.0 mol of Al are produced?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2


Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1
You know the right answer?
Using the balanced chemical equation below. 2Al2O3 --> 4Al + 3O2 How many moles of oxygen are pro...
Questions


Spanish, 19.05.2021 18:00



Mathematics, 19.05.2021 18:00

Physics, 19.05.2021 18:00

Business, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00





Chemistry, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00

Spanish, 19.05.2021 18:00

Biology, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00