
Chemistry, 09.01.2021 02:20 serenityjohnson98765
The structural formula for two isomers of 1, 2-dichloroethene are shown above. which of the two liquids has the higher equilibrium vapor pressure at 20° C and why
a) the cis-omer because it only has dipole dipole and the trans-iomer has london dispersion forces
b) cis-omer because it has only ldf and trans-iomer has dipole dipole
c) trans-iomer because it has only dipole dipole and cis-omer has ldf
d) trans-iomer because it has only ldf and cis-omer has dipole dipole
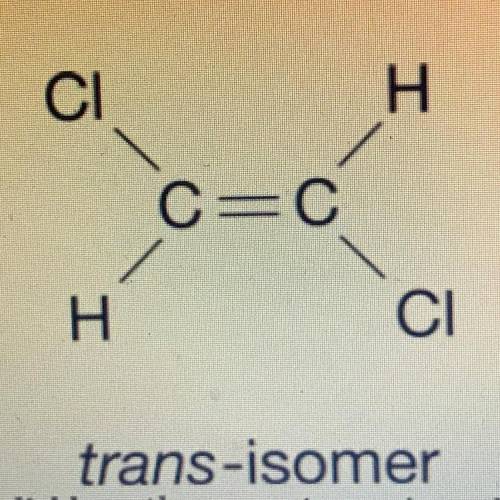

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 3

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
The structural formula for two isomers of 1, 2-dichloroethene are shown above. which of the two liqu...
Questions



Mathematics, 19.06.2020 10:57




Mathematics, 19.06.2020 10:57


Mathematics, 19.06.2020 10:57






Chemistry, 19.06.2020 10:57


Computers and Technology, 19.06.2020 10:57



English, 19.06.2020 10:57



