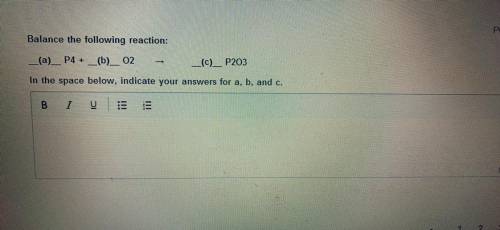
Balance the following reaction in the space below indicate your answer for a b and c please
...


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

You know the right answer?
Questions

Geography, 15.06.2021 14:00

Physics, 15.06.2021 14:00


Mathematics, 15.06.2021 14:00


Mathematics, 15.06.2021 14:00

Social Studies, 15.06.2021 14:00



Mathematics, 15.06.2021 14:00



English, 15.06.2021 14:00


Mathematics, 15.06.2021 14:00



Computers and Technology, 15.06.2021 14:00


Biology, 15.06.2021 14:00