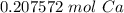How many moles of Ca are in 1.25x1023 atoms?
We used two conversion factors in this unit, they are provided below. It is your responsibility to know which conversion factor to use!
Avogadro’s number: 6.02x1023 atoms = 1 mole
Molar mass of calcium: 40.078 g Ca = 1 mol Ca
A. 3.12x10^21 mol
B. 0.21 mol
C. 3.12 mol
D. 0.21x10^21 mol

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

Chemistry, 23.06.2019 12:10
What is the correct name for hg(no3)2? mercury (i) nitrate mercury (ii) nitrate mercury nitroxide mercury dinitride
Answers: 1
You know the right answer?
How many moles of Ca are in 1.25x1023 atoms?
We used two conversion factors in this unit, they are...
Questions

Biology, 21.09.2019 03:30

English, 21.09.2019 03:30

History, 21.09.2019 03:30

Mathematics, 21.09.2019 03:30

Biology, 21.09.2019 03:30

Biology, 21.09.2019 03:30



Biology, 21.09.2019 03:30



Mathematics, 21.09.2019 03:30



Business, 21.09.2019 03:30


Mathematics, 21.09.2019 03:30

Mathematics, 21.09.2019 03:30


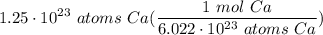 Multiply:
Multiply: