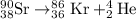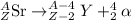Chemistry, 10.01.2021 08:50 kenziepickup
9. Write a balanced nuclear equation for the following: The isotope Strontium-90 decays by Q-
decay (1 point)
OfSr — He +56 kr
OS → H +39 kr
o Sr + Be +38kr
99 Sr → He +3 Se
SAST +7+99 Sr

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1


Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
9. Write a balanced nuclear equation for the following: The isotope Strontium-90 decays by Q-
decay...
Questions

Mathematics, 24.04.2020 21:08



Health, 24.04.2020 21:08










Mathematics, 24.04.2020 21:23


Biology, 24.04.2020 21:23

Mathematics, 24.04.2020 21:23

Mathematics, 24.04.2020 21:23

English, 24.04.2020 21:23