
Nitrogen dioxide can be made with the reaction of nitrogen monoxide and oxygen.
Assuming that the reaction is at equilibrium, what effect would a decrease in the concentration of NO_2 have on the equilibrium position once the equilibrium is reestablished?
A. An increase in the amount of begin mathsize 14px style NO subscript 2 end style.
B. An increase in the amount of begin mathsize 14px style straight O subscript 2 end style.
C. No change in the amount of begin mathsize 14px style straight O subscript 2 end style.
D. An increase in the amount of NO.
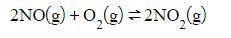

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3


Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
Nitrogen dioxide can be made with the reaction of nitrogen monoxide and oxygen.
Assuming that the r...
Questions

Mathematics, 20.05.2021 05:20

Biology, 20.05.2021 05:20


English, 20.05.2021 05:20




Mathematics, 20.05.2021 05:20


Mathematics, 20.05.2021 05:20


Mathematics, 20.05.2021 05:20

English, 20.05.2021 05:20

Health, 20.05.2021 05:20




Mathematics, 20.05.2021 05:20




