
Chemistry, 10.01.2021 22:20 Itsyourgirllulu
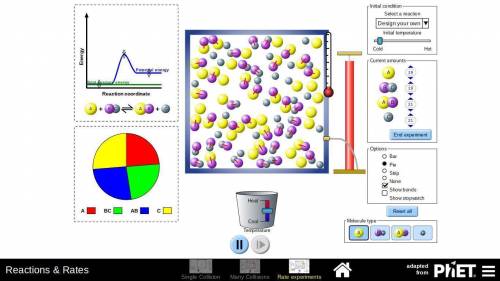
In part D of task 1, you identified at least two ways in which the reaction of nitrogen and hydrogen could be changed to alter the equilibrium. Use the simulation to test those changes. Describe how you used the simulation to model the changes and the results they produced. Use these methods if you find them helpful:
Look at the pie graph to see how the system changes.
Use the Temperature slider at the bottom to cool or heat the mixture.
Click the pause button on the simulation to observe the number of particles at any point of time.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2


Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
In part D of task 1, you identified at least two ways in which the reaction of nitrogen and hydrogen...
Questions

Spanish, 03.03.2021 01:40

Mathematics, 03.03.2021 01:40

Biology, 03.03.2021 01:40

Chemistry, 03.03.2021 01:40

Physics, 03.03.2021 01:40

Computers and Technology, 03.03.2021 01:40

Biology, 03.03.2021 01:40

Arts, 03.03.2021 01:40

Mathematics, 03.03.2021 01:40




English, 03.03.2021 01:40

Mathematics, 03.03.2021 01:40

Mathematics, 03.03.2021 01:40

Mathematics, 03.03.2021 01:40

History, 03.03.2021 01:40

Mathematics, 03.03.2021 01:40




