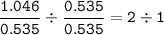Chemistry, 11.01.2021 02:50 samantha636
66.41 g of Copper [Cu] are combusted with 8.56g of Oxygen [O] to produce a compound. What is the empirical formula of the compound?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Hannah is writing a report on how albedo affects the global climate. she’s proofreading her passage for any factual errors. which sentence must hannah correct before submitting her report? earth receives energy from the sun. this energy drives many of the processes on earth, including its climate. some part of this energy is reflected by earth’s surface. we use the term albedo to describe the reflected energy. albedo of an object is the ratio of the reflected radiation to the total radiation reaching the object. a value of 0 means no energy is absorbed by the object, whereas a value of 1 means that all of the energy is absorbed. in this way, the albedo of an object can influence earth’s atmospheric temperature.
Answers: 1


Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
66.41 g of Copper [Cu] are combusted with 8.56g of Oxygen [O] to produce a
compound. What is the em...
Questions

Computers and Technology, 15.09.2021 20:10

Biology, 15.09.2021 20:10



Mathematics, 15.09.2021 20:10

History, 15.09.2021 20:10


Biology, 15.09.2021 20:10

Mathematics, 15.09.2021 20:10

English, 15.09.2021 20:10


Geography, 15.09.2021 20:10




Mathematics, 15.09.2021 20:10

History, 15.09.2021 20:10


Chemistry, 15.09.2021 20:10