
Chemistry, 11.01.2021 03:40 qveenjordan6456
Suppose 275 g of NO3- flows into a swamp each day. What volume of CO2 would be produced each day at 17.0°C and 1.00 atm? I attached the image of the equation for reference


Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
Suppose 275 g of NO3- flows into a swamp each day. What volume of CO2 would be produced each day at...
Questions



History, 17.11.2020 22:20



Biology, 17.11.2020 22:20


History, 17.11.2020 22:20



Mathematics, 17.11.2020 22:20

Mathematics, 17.11.2020 22:20

Mathematics, 17.11.2020 22:20







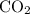 produced has been 264.28 L.
produced has been 264.28 L.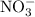 has been:
has been: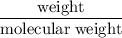
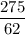
 Volume = moles
Volume = moles 

