
Chemistry, 11.01.2021 04:10 makennahudson94
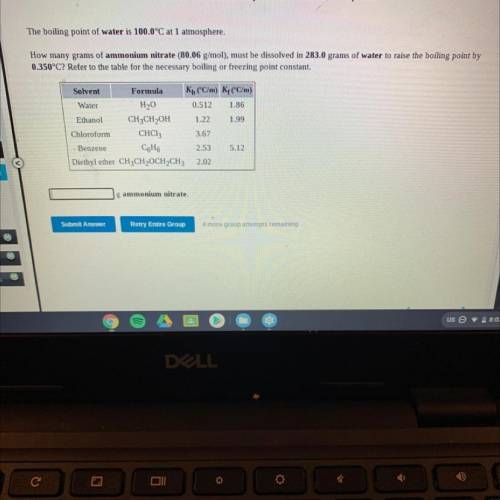
The boiling point of water is 100.0°C at 1 atmosphere.
How many grams of ammonium nitrate (80.06 g/mol), must be dissolved in 283.0 grams of water to raise the boiling point by
0.350°C? Refer to the table for the necessary boiling or freezing point constant.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1
You know the right answer?
The boiling point of water is 100.0°C at 1 atmosphere.
How many grams of ammonium nitrate (80.06 g/...
Questions

Mathematics, 22.01.2020 08:31


Mathematics, 22.01.2020 08:31

Mathematics, 22.01.2020 08:31

Mathematics, 22.01.2020 08:31


Mathematics, 22.01.2020 08:31

History, 22.01.2020 08:31

Mathematics, 22.01.2020 08:31

History, 22.01.2020 08:31


Computers and Technology, 22.01.2020 08:31


History, 22.01.2020 08:31

Mathematics, 22.01.2020 08:31

Mathematics, 22.01.2020 08:31

Mathematics, 22.01.2020 08:31


Health, 22.01.2020 08:31



