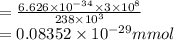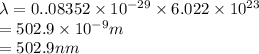Chemistry, 11.01.2021 15:40 vanessacox45
It takes to break a carbon-iodine single bond. Calculate the maximum wavelength of light for which a carbon-iodine single bond could be broken by absorbing a single photon.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
It takes to break a carbon-iodine single bond. Calculate the maximum wavelength of light for which a...
Questions



English, 03.02.2021 18:00

English, 03.02.2021 18:00

History, 03.02.2021 18:00

Social Studies, 03.02.2021 18:00

Mathematics, 03.02.2021 18:00


Biology, 03.02.2021 18:00



Mathematics, 03.02.2021 18:00




Mathematics, 03.02.2021 18:00

English, 03.02.2021 18:00