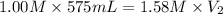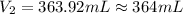Chemistry, 11.01.2021 16:10 makailaaa2
A chemist must prepare 575.mL of 1.00M aqueous sodium carbonate Na2CO3 working solution. He'll do this by pouring out some 1.58M aqueous sodium carbonate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in mL of the sodium carbonate stock solution that the chemist should pour out. Round your answer to 3 significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:10
How many grams of naoh are needed to make 0.250 liter of a 0.500 m solution of naoh? 0.125 g 5.00 g 2.00 g
Answers: 1

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1
You know the right answer?
A chemist must prepare 575.mL of 1.00M aqueous sodium carbonate Na2CO3 working solution. He'll do th...
Questions


Mathematics, 31.07.2019 19:00



Mathematics, 31.07.2019 19:00

Mathematics, 31.07.2019 19:00

Business, 31.07.2019 19:00

History, 31.07.2019 19:00



English, 31.07.2019 19:00

Biology, 31.07.2019 19:00


History, 31.07.2019 19:00

Biology, 31.07.2019 19:00

Social Studies, 31.07.2019 19:00

Social Studies, 31.07.2019 19:00

Business, 31.07.2019 19:00


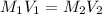
 = molarity of aqueous sodium carbonate
= molarity of aqueous sodium carbonate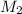 = molarity of aqueous sodium carbonate stock solution
= molarity of aqueous sodium carbonate stock solution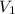 = volume of aqueous sodium carbonate
= volume of aqueous sodium carbonate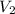 = volume of aqueous sodium carbonate stock solution
= volume of aqueous sodium carbonate stock solution