Which of the following statements apply to an ionic bond?
Check all that apply.
A. It is made...

Chemistry, 11.01.2021 17:30 Austin4094
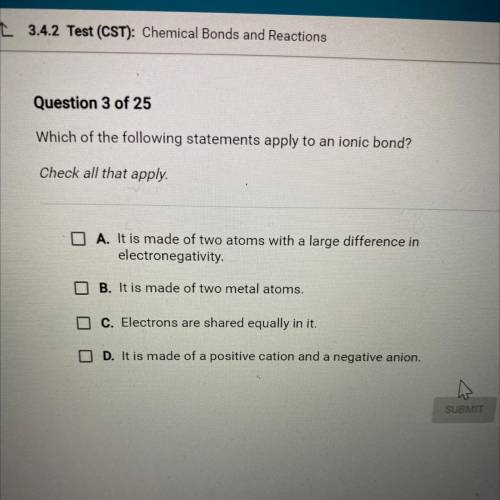
Which of the following statements apply to an ionic bond?
Check all that apply.
A. It is made of two atoms with a large difference in
electronegativity.
B. It is made of two metal atoms.
C. Electrons are shared equally in it.
D. It is made of a positive cation and a negative anion.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2


Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Questions

Social Studies, 18.02.2022 14:00



Spanish, 18.02.2022 14:00


Spanish, 18.02.2022 14:00


Biology, 18.02.2022 14:00





Mathematics, 18.02.2022 14:00


Mathematics, 18.02.2022 14:00

Advanced Placement (AP), 18.02.2022 14:00




Business, 18.02.2022 14:00



